Vapor-compression refrigeration cycle
engineering , thermodynamicsThe vapor-compression refrigeration cycle uses a carefully chosen refrigerant substance to transfer heat from the inside of the box to the surroundings. It uses four devices to make it happen. I will describe the cycle from the throttle, but since it’s a cycle any other starting point is valid. Many explanations of the vapor-compression cycle use the expressions “high/low pressure, high/low temperature” (HP, LP, HT, LT) without saying compared to what, causing confusion. I tried to clarify the use of those expressions every time there might be some doubt.
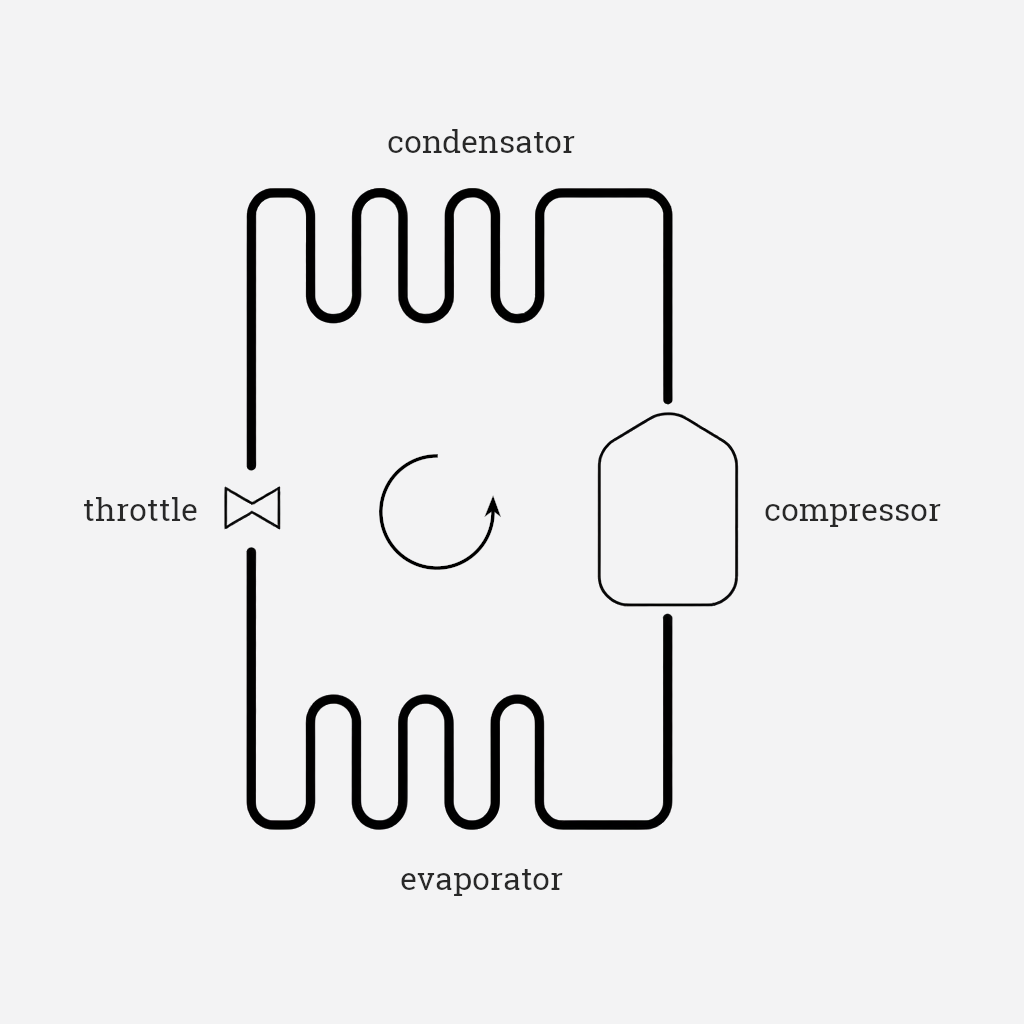
The refrigerant, now liquid, goes through a throttle. The throttle uses a pressure difference to expand the refrigerant: coming in the throttle, the refrigerant is high pressure (higher than the other end of the throttle), low temperature (ambient temperature), liquid; due to the lower pressure on the other side of the throttle, the refrigerant expands (and thus it cools down a bit). Due to the pressure drop, the boiling point1 of the refrigerant also drops significantly, and the new temperature of the substance is still higher than this new boiling point; as a consequence, the refrigerant partially evaporates. This phase change absorbs heat from the refrigerant, causing it to cool down significantly. The refrigerant comes out as a low pressure, low temperature mixture of liquid and vapor (mostly liquid).
This mixture goes through the evaporator; this is a passive heat exchanger: it is a surface area between the refrigerant and the content of the box that needs to be cooled down. The refrigerant mixture is very cold from the previous step (colder than the inside of the box), so it takes heat away from the inside. This is the step where the actual cooling action takes place. The refrigerant being at a low pressure ensures that its boiling point is very low, so the heat absorbed from the box is enough to completely evaporate it, hence the name of the component. In this step, the refrigerant undergoes a phase change from liquid to vapor; the heat absorbed from the box is stored as latent heat of evaporation2 and it does not cause an increase in temperature. The refrigerant comes out of the evaporator as a low pressure, low temperature vapor. Note: the refrigerant needs to be completely evaporated when it comes out of the evaporator, to avoid damaging other components; to ensure this, the refrigerant actually gets slightly super-heated (meaning that its temperature is intentionally increased slightly beyond its boiling point at the current pressure). This process is not strictly part of the vapor-compression cycle, but it is crucial for the correct functioning of the device.
The refrigerant goes through the compressor. The compressor does work to increase the pressure of the refrigerant, and thus its temperature, and it comes out as a high pressure, high temperature vapor. The compressor is the only one of the four components that requires an energy input. Note that the increase in pressure also causes an increase in the refrigerant’s boiling point: this is crucial for the next step (the refrigerant can stay liquid at a higher temperature).
The refrigerant goes through the condenser; this is another heat exchanger, but this time the refrigerant loses its latent heat to the surroundings. In doing so, it condenses and goes back to being a high pressure, low temperature (ambient temperature) liquid, and it can re-enter the throttle.
Summary
Throttle
The refrigerant expands due to pressure drop, causing a significant temperature drop.
Evaporator
The refrigerant absorbs heat from the inside of the box, evaporating at constant temperature.
Compressor
Work is done on the refrigerant to increase its pressure, and thus its temperature and its boiling point. If the boiling point stayed low, the ambient temperature would be enough to keep the refrigerant a vapor.
Condenser
The refrigerant loses heat to the surroundings and turns back into a liquid.